Gentaur HIV replication products are available at Genprice USA from different suppliers at the best prices. Our products are of very high quality, validated by competent laboratories for exclusive use in research. You can order online or send your order to your local contact. If you are not sure of the product choice required for your research, you can always request a selection audit.
Summary
Inhibition of HIV replication initially focused on viral enzymes, which are exclusively expressed by the virus and are not present in the human cell. The development of reverse transcriptase (RT) inhibitors began with the discovery of the antiretroviral activity of the nucleoside analogue zidovudine in March 1987. Currently, six main classes of antiretroviral drugs are used for the treatment of HIV-infected patients: RT inhibitors, nucleoside and non-nucleoside inhibitors, protease inhibitors, the integrase inhibitors raltegravir, the fusion inhibitor enfuvirtide (T-20), and the 5-chemokine receptor antagonist maraviroc.
A seventh class, the maturation inhibitors, have not yet been approved because their efficacy is affected by HIV-1 polymorphisms that occur naturally in 30-40% of untreated HIV-1 isolates. The use of antiretroviral combination therapy has been shown to be effective in slowing the progression to AIDS and in reconstituting the immune system of people infected with HIV. Over the past 5 years, the introduction of newer antiretrovirals has greatly increased the effectiveness of treatment. However, the development and accumulation of resistance to all classes of antiretroviral drugs remain a major problem. Additional goals will need to be defined to achieve the ultimate goal: the eradication of the virus from the infected human body.
Reverse transcription inhibitors
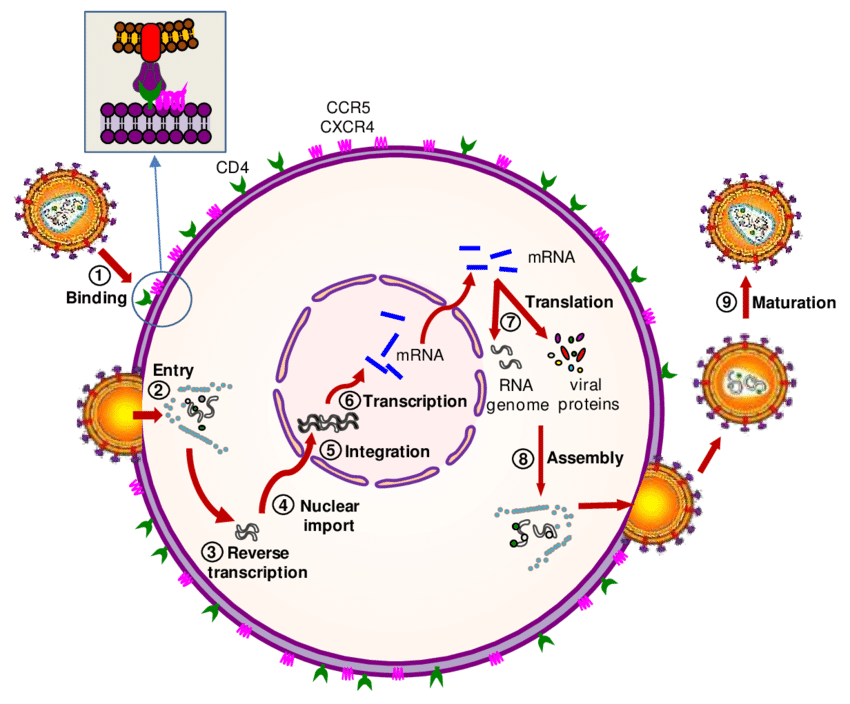
After the release of the capsid into the cytoplasm, the capsid and nucleocapsid disassemble (mismatch), although the precise mechanism is still unknown. Genomic RNA is associated with viral tRNALys and with several viral proteins such as RT, IN, PR, Vpr, and MA that constitute the reverse transcription complex (RTC). The RTC uses the microtubule system for transport through the cytoplasm. Within the RTC, reverse transcription of viral RNA into DNA occurs by viral RT, although the efficiency of reverse transcription is highly dependent on the presence of all components of the RTC. For example, in the absence of the IN protein, reverse transcription is completely blocked.
RT is an RNA-dependent DNA polymerase that produces double-stranded DNA from single-stranded RNA. This process begins with the synthesis of negatively oriented single-stranded DNA copied from viral RNAs, which is used as a template for subsequent second-strand DNA synthesis. RT is a heteromeric enzyme that comprises a regulatory subunit (p51) and a catalytic subunit (RNase H – p15) that form the p66 molecule. p66 resembles a right hand, where the subdomains are designated fingers, palm, and thumb. The catalytic site is located in the palm and comprises amino acids D185-D186 and D110, a highly conserved motif also in other RTs and polymerases.
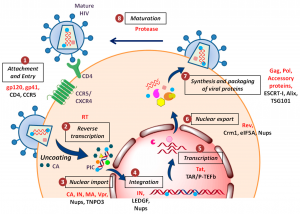
It includes the activity of viral ribonuclease H, responsible for the degradation of the template RNA of the DNA/RNA hybrid. Since HIV-1 RT is reported not to maintain sustained replication for more than about 100 to 200 bases, reverse transcription is the replication step with the highest probability of recombination events between the two HIV-RNA strains. 1 in each particle. Like all RNA polymerases, HIV RT has a high error rate when transcribing RNA into DNA, as it has no proofreading ability. This high error rate, in combination with the high rate of recombination, allows mutations to accumulate at a rapid rate, which has important implications for immune escape, development of drug resistance, and tropism switching, among others.
IN inhibitors
Different substances are currently being developed, but only one licensed for clinical use belongs to the so-called chain transfer inhibitors. These drugs bind to IN near the DDE motif in the active site and competitively block IN activity. Proviral DNA cannot be inserted into the host genome and is circularized by cell repair enzymes, irreversibly stopping viral replication.
Raltegravir (RAL, Isentress®, Merck) is a chain transfer inhibitor with potent activity against HIV-1 and HIV-2. RAL is administered orally twice daily, does not require RTV boosting, and is well tolerated. Results from clinical trials indicate that RAL is safe and highly effective in the treatment of antiretroviral treatment-naïve and antiretroviral treatment-experienced patients. Resistance to RAL has been associated with amino acid substitutions in three key positions of the IN protein: Y143R/C, Q148H/R/K or H155H, alone or accompanied by other mutations such as T66I, L74M, E92Q, T97A, E138K+ G140S/ To GY143H, V151I and G163R.
Elvitegravir (EVG, GS-9137, Gilead), a second-chain transfer inhibitor, is in phase III clinical trial. It is also active against HIV-1 and HIV-2. EVG has the advantage of once-daily oral dosing when boosted with RTV. EVG resistance is associated with mutations T66I/A/K, E92Q, E138K, Q146P, S147G, Q148R/H/K, and N155H, which are close to RAL-selected resistance mutations; therefore, cross-resistance for both drugs is expected. Other drugs currently in development are reviewed in Serrao et al.
Maturation inhibitors
Maturation inhibitors are drugs that target one or more cleavage sites within Gag precursor proteins or that inhibit the capsid protein interactions required for core condensation. Bevirimat (PA-457; Myriad Pharmaceuticals) is the first compound in its class, although the drug has not yet been approved by the FDA and the EMEA. Bevirimat is inactive against HIV-2.
HIV-1 mutations conferring resistance to bevirimat were located at the P24/P2 cleavage site (H358Y, L363M/F, A364I/M/V, and A366V/T) and at the P2 peptide (Q369H, V370A/M/ del and T371del), either by increasing the rate of cleavage at the P24/P2 site by viral PR or by interfering with drug binding. Unfortunately, the efficacy of bevirimat therapy is affected by HIV-1 polymorphisms at P2 (amino acids 369–371), which occur naturally in 30–40% of treatment-naïve isolates of HIV-1. In addition, co-evolution of HIV PR and Gag mutations has been observed during PI exposure, and PI treatment failures increase the prevalence of bevirimat resistance and reduce clinical outcomes during bevirimat therapy.
Conclusions
The use of combination antiretroviral therapy has been shown to be effective against progression to AIDS in HIV-infected individuals. Over the past 5 years, the introduction of two new PIs (DRV and TPV) with broad activities against PI-resistant viral strains, the CCR5 antagonist maraviroc, the IN inhibitor RAL, and the second-generation NNRTI ETR, have greatly increased the efficacy of the drug. antiretroviral treatment. Meanwhile, successfully treated HIV infection can be considered a chronic disease rather than a fatal infection.
However, the success of antiretroviral therapy is limited by high costs, the development of viral resistance, and side effects. The eradication of the virus from the infected body by combination antiretroviral therapy or the cure of HIV infection is still not possible. Additional goals will need to be defined to achieve the goal of medical intervention in HIV infection: a global perspective for surviving HIV infection to normal life expectancy.